MEDICATION PRESCRIPTION REVIEW PROCESS
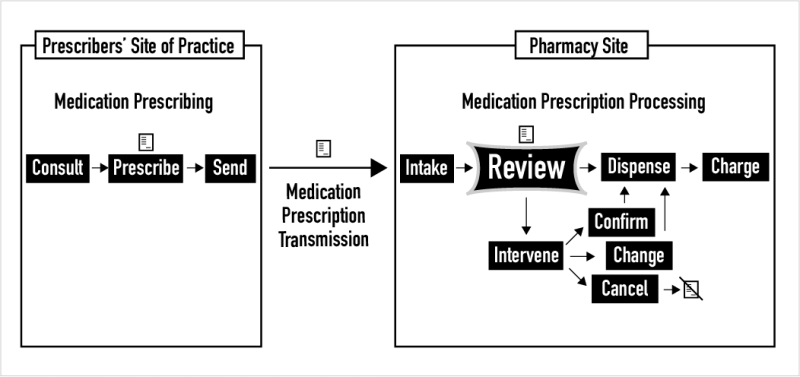
The task of medication prescription review is an independent double-check. It is a task that occurs in all pharmacies after new medication prescriptions (also called orders) are taken in from prescribers. As shown above, after intake, the review task unfolds and ends with the dichotomous choice of dispensing a drug product or intervening to confirm, change, or cancel a medication prescription due to any issues found. This website focuses on the task and process of new prescription review in pharmacies. In the United States in 2020, more than 6 billion medication prescriptions were dispensed, including refills. An additional billion or more new medication orders are created annually for inpatients in America’s 6000 hospitals. So, just for new prescriptions, this task is performed approximately 3 billion times a year by the 315,000 pharmacists licensed to practice in the U.S.
THE END OF PAPER PRESCRIPTIONS

Legal medication prescriptions must have certain data elements and be signed by the prescriber. The most basic data elements include the patient’s name, date, medication name, and the quantity of dosage forms (e.g., tablets or capsules) to be dispensed. While most medication prescriptions are now electronic and not written on paper, still only a tiny amount of basic information, often conveyed using medical jargon, is required for a medication prescription to be legal. Of course, with paper and pen or electronically, it is possible to create much more elaborate medication prescriptions, as prescribers sometimes do.
PRESCRIPTION REVIEW TASK MODEL

During the process of medication prescription review, two predictions are made. Once those two predictions have been made and then also interpreted to make intermediate decisions about safety and likely efficacy, these intermediate decisions are combined to finally decide whether to dispense a drug product for a patient or to intervene. When a decision to dispense or intervene is reached, the medication prescription review task is complete. This task model was formalized with the help of version 1.3 of the Decision Model and Notation (DMN) from the Object Management Group.
POTENTIAL FOR PARTIAL AUTOMATION

A deeper understanding of the task of new prescription review in pharmacies, and especially of the information and knowledge needed to do the task successfully, could lead to its partial automation. The automation that may come will necessarily be partial since it is clear that there are medication prescriptions that should always be reviewed in the manual clinical way by expert pharmacists. For example, for nearly all chemotherapy prescriptions, pharmacists carefully compare the prescriptions for chemo to previously published evidence-based regimens describing cancer treatment. In the future, pharmacists would have more time to focus on reviewing the most complex and risky prescriptions If some other prescription reviews can be safely automated.
NEED FOR PROCESS FORMALIZATION
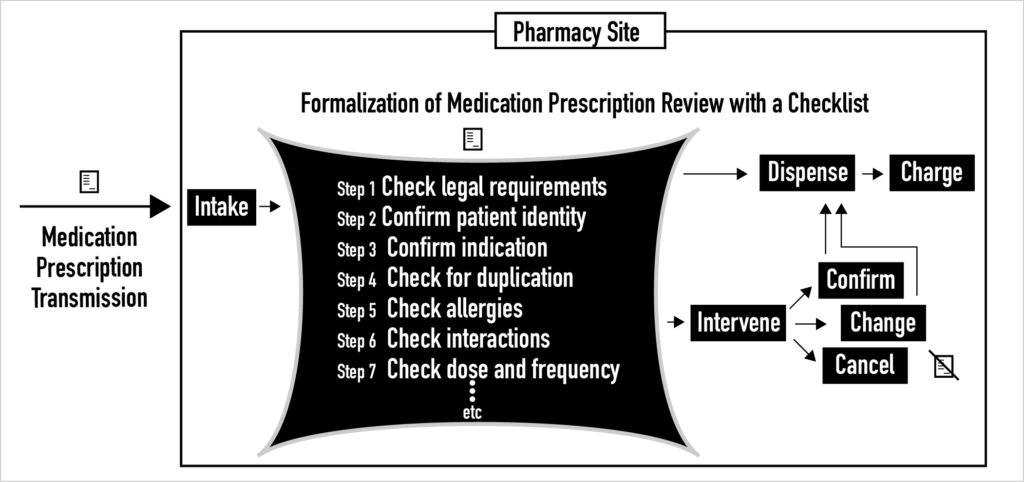
Inside pharmacies today, for complex socio-cultural reasons, the current process of clinical medication prescription review is highly variable and unformalized. For example, checklists are rarely used to guide the prescription review process. Instead, with a few notable exceptions like FDA REMS, printed and online policies and procedures provide only general guidance to pharmacists about how to do this review task. Over the last decade, small steps have been taken that begin to formalize the process of clinical medication prescription review. In the future, whether medication prescription review is done clinically by pharmacists or statistically by machines, the process of prescription review must be formalized by specifying sequences of steps with corresponding checklists. Otherwise, it is unlikely that needed safety and quality gains will be achieved. It is unlikely because safety and quality gains are known to be a function of process formalization and standardization so long as flexibility and adaptability in rare and unexpected cases is maintained.
ELECTRONIC PRESCRIPTION QUEUES
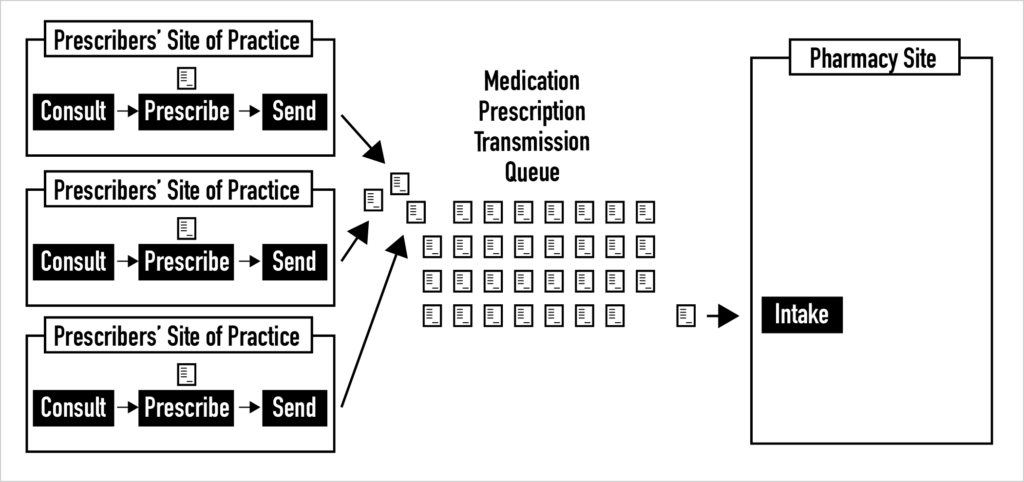
All pharmacies have queues of electronic prescriptions coming from various sites of practice that are waiting to be reviewed. These queues are not well understood. Unfortunately, some of the most basic metadata about each electronic medication prescription is not conveyed with the prescriptions. For example, pharmacists receive little or no information about whether a prescription was selected by the prescriber from a list of “pre-built” or scripted prescriptions, whether it stemmed from using an order set, or whether it was created as a custom prescription. In addition, we have no idea about the risk mix of the electronic prescriptions in these queues and little insight into e-prescription queue dynamics. Pharmacy-level prescription queue analysis that leads to enriching the information about electronic prescriptions sent to pharmacies is desperately needed.