TEN TECHNICAL CAPABILITIES
To make statistical medication prescription review (SMPR) possible, a new technical information processing system has to be invented. Since no such technical system has been built yet, one can imagine a variety of technical development approaches that could be taken to develop it. In all cases, SMPR systems will be complex and involved. For this reason, significant technical R&D is needed to develop and evaluate the first generation of SMPR systems.
BASIC FUNCTION OF AN SMPR SYSTEM
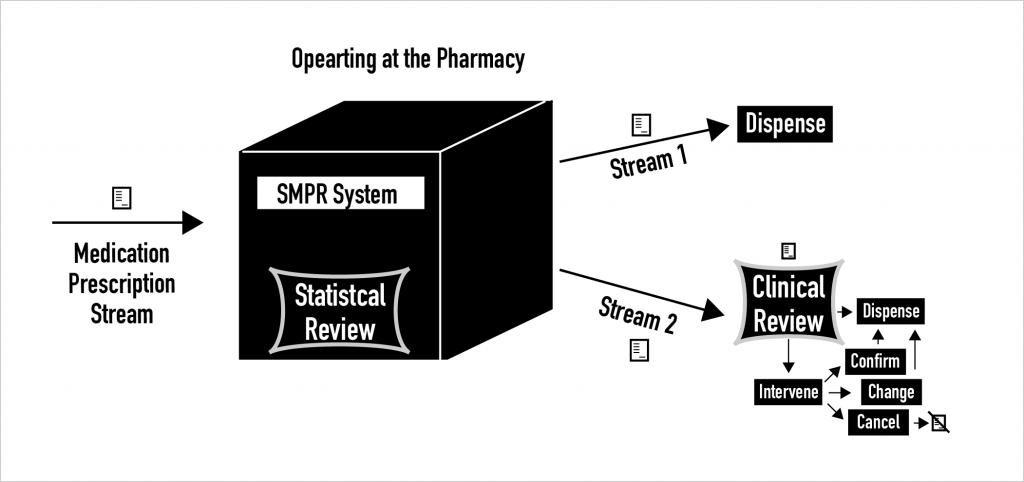
A statistical medication prescription review (SMPR) system separates a stream of new electronic medication prescriptions (or medication orders) received at the pharmacy into two very different streams.
After SMPR takes place, a new Stream 1 carries the electronic medication prescriptions that are categorized by SMPR as safe and potentially effective.
Meanwhile, a new Stream 2 shuttles all other electronic medication prescriptions – and the added results from SMPR – on to pharmacists for clinical medication prescription review.
By separating the incoming medication prescription stream into two, SMPR systems offer the potential to make better use of limited pharmacist work time by decreasing the number of prospective clinical medication prescription reviews that pharmacists must do.
This basic SMPR setup is shown in the following figure.
What does the SMPR system “black box” in the figure actually do when it processes new electronic medication prescriptions? To begin to answer that question, what follows is a list and then descriptions of the major technical capabilities that any SMPR system must have.
LIST OF TEN CAPABILITIES
- Prescription Risk Stratification
- Prescription Complexity Stratification
- Prescription Information Quality Checking
- Patient Medication Exposure History Analysis
- Medication Prescription Anomaly Detection
- Prescription and Patient Profile Fit Assessment
- Prescriber Background and Prescribing Experience Analysis
- Production Rule Processing for Allergies, Drug-Drug Interactions, etc.
- Patient-Specific Dose Calculation
- Positive and Negative Outcomes Prediction
1) PRESCRIPTION RISK STRATIFICATION
First and foremost, every new electronic medication prescription (or medication order) needs to be categorized by the SMPR system according to its apparent risk, which means it must be risk stratified in a one or more ways.
Why is prescription-level risk estimation, scoring, categorization, and stratification so important? Because at the outset of work on SMPR systems, it seems that risk stratification holds the greatest potential to modulate and exert user control over SMPR systems.
With high-accuracy prescription risk stratification, users will be able to set their own risk threshold above which pharmacist clinical medication prescription review is always mandatory.
2) PRESCRIPTION COMPLEXITY STRATIFICATION
In a manner that is similar to risk stratification, individual electronic medication prescriptions can also be scored for complexity. Complexity scores account for the dosage form and frequency of daily use, among other factors.
SMPR system users will also be able to set their own complexity threshold above which pharmacist clinical medication prescription review is always mandatory.
3) PRESCRIPTION INFORMATION QUALITY CHECKING
SMPR systems need to use natural language processing (NLP) and other analytic techniques to check and score the quality and completeness of the information in each electronic medication prescription.
Electronic medication prescriptions that are incomplete or ambiguous must be flagged and put into Stream 2 for further clinical medication prescription review by pharmacists.
4) PATIENT MEDICATION EXPOSURE HISTORY ANALYSIS
A challenge for SMPR system developers will be to source patient-level medication exposure history information for analysis. It matters whether a new prescription specifies a medication that the patient has used many times, has rarely used, or has never used before. What happened when a patient was previously exposed to medication, for better and worse, also matters.
The initial goal of patient medication exposure history analysis is to determine which medication prescriptions are customary for the patient, and which others are new and being prescribed for the first time.
To begin, the patient medication exposure history analyzer must be capable of identifying all medication prescriptions that would result in first-ever medication exposures. Soon after, a capabilities to identify what is customary and to calculate total lifetime exposures to certain prescription medications should be added. Even more advanced past medication use outcomes analyses should be included as SMPR systems evolve.
5) MEDICATION PRESCRIPTION ANOMALY DETECTION
Medication prescriptions can be odd, strange, or rare in multiple different ways. SMPR systems must detect this.
Anomaly detection begins by comparing the details of electronic medication prescriptions to past medication prescribing patterns for similar indications or patients. More advanced anomaly detection will take account of institutional, local or regional, and national prescribing patterns.
6) PRESCRIPTION AND PATIENT PROFILE FIT ASSESSMENT
SMPR systems must detect when a new medication prescription is and is not expected given the EHR record and overall profile of the patient who will be exposed to the newly prescribed medication. This kind of analytic can be thought of as computing the “fit” between the new electronic medication prescription and the patient for whom the prescription is written.
7) PRESCRIBER BACKGROUND AND PRESCRIBING EXPERIENCE ANALYSIS
SMPR systems must check the degree to which a prescriber and their medical speciality suggest prescriber familiarity with and expertise using the medication prescribed in a new prescription.
With sufficient prescriber-level prescription data, like the data used by the pharmaceutical industry for marketing purposes, SMPR systems can tell the difference between a new medication prescription specifying a medication that the prescriber uses a lot versus a prescription for a medication the prescriber rarely if ever uses. For the latter, a follow-on clinical medication prescription review by a pharmacist may be more desirable.
8) PRODUCTION RULE PROCESSING FOR ALLERGIES, INTERACTIONS, ETC.
Like other clinical decision support systems for medications, SMPR systems require sophisticated rules engines backed by comprehensive drug knowledge bases.
SMPR systems must have rules to do allergy checks, drug-drug, drug-food, and drug-disease interaction checks, pharmacogenetic drug-gene checks, and other checks besides.
SMPR systems must be capable of applying domain-specific rule sets for domains like infectious disease, pain management, and others. SMPR systems must also be able to apply medication prescribing policy rules, e.g., rules governing the number of refills allowed and things like that.
9) PATIENT-SPECIFIC DOSE CALCULATION
As the number of determinants of a safe patient-specific dose for a medication increase, SMPR systems will need to account for these determinants and independently compute possible doses and dose ranges from patient information for comparison with doses given in new electronic medication prescriptions.
10) POSITIVE AND NEGATIVE OUTCOMES PREDICTION
To judge likely safety and potential efficacy, SMPR systems must have capabilities to predict a large number of different positive and negative medication use outcomes.
For medications where use is guided and therapeutic gain can be determined by objective outcome measures, e.g. measures of blood pressure, glucose, or cholesterol, SMPR systems must have reliable, well-calibrated predictive models capable of predicting the effect of prescribed medicines on these objective measures.
Similarly, for medications where adverse drug events can be predicted based on patient information, SMPR systems must have reliable, well-calibrated predictive models capable of predicting adverse drug events and thereby causing them to be recognized as early as possible if not altogether averted.